Redness Reliever Eye Drops
LUMIFY
LUMIFY redness reliever eye drops significantly reduce redness to help eyes look whiter and brighter, revealing your eyes’ natural beauty. Works in 1 minute.
BUY NOW
Unique patented formulation

Clinically tested
#1 Eye doctor recommended redness reliever eye drop
- Unique, FDA-approved formulation uses low-dose brimonidine to selectively target redness
- Works in just 60 seconds and lasts up to 8 hours
- Lower risk of side effects like rebound redness when used as directed
- Absolutely no bleach or dyes
- #1 eye doctor recommended redness reliever eye drop
Active ingredient: Brimonidine tartrate (0.025%) - redness reliever
Inactive ingredients: Benzalkonium chloride, boric acid, calcium chloride dihydrate, glycerin, potassium chloride, sodium borate decahydrate, sodium chloride, water for injection. Hydrochloric acid and/or sodium hydroxide may be used to adjust pH.
Drug FactsRelieves redness of the eye due to minor eye irritations
Adults and children 5 years of age and over:
- Instill 1 drop in the affected eye(s) every 6-8 hours
- Do not use more than 4 times daily
- Remove contact lenses before use
- Wait at least 10 minutes before re-inserting contact lenses after use
- If using other ophthalmic products while using this product wait at least 5 minutes between each product
- To avoid contamination, do not touch tip of container to any surface
- Replace cap after each use
- Children under 5 years of age: consult a doctor
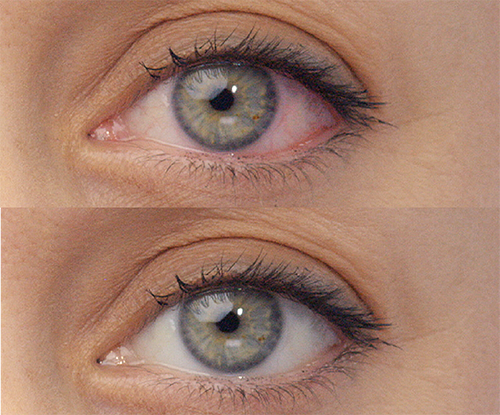
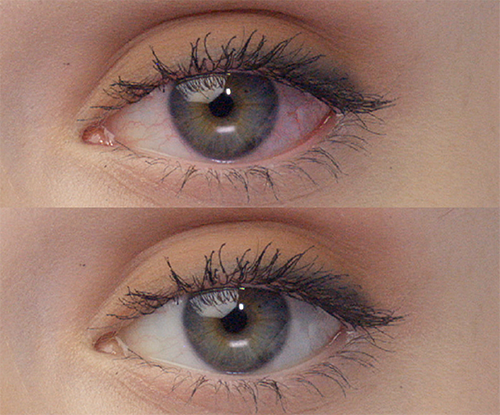
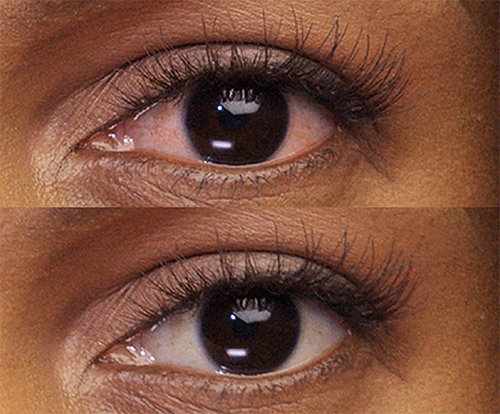
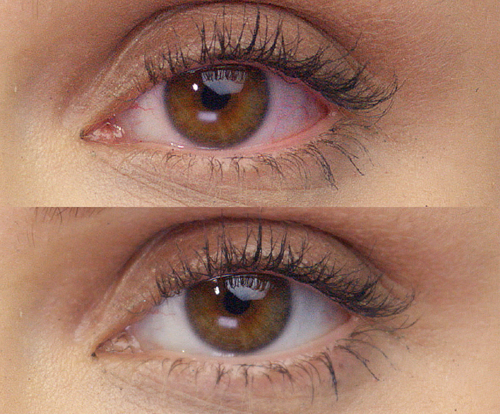
REAL PEOPLE,
REAL RESULTS.
People can’t stop sharing how amazing their eyes look with LUMIFY eye drops! See for yourself why LUMIFY is a redness reliever like no other.*
SIGNIFICANTLY REDUCE REDNESS
TO HELP EYES LOOK WHITER AND BRIGHTER
LASTS UP TO 8 HOURS
SHOP OTHER PRODUCTS
DISCOVER MORE

























